Engineered Virus Crosses Blood-Brain Barrier, Delivers Therapeutic Genes in Mice
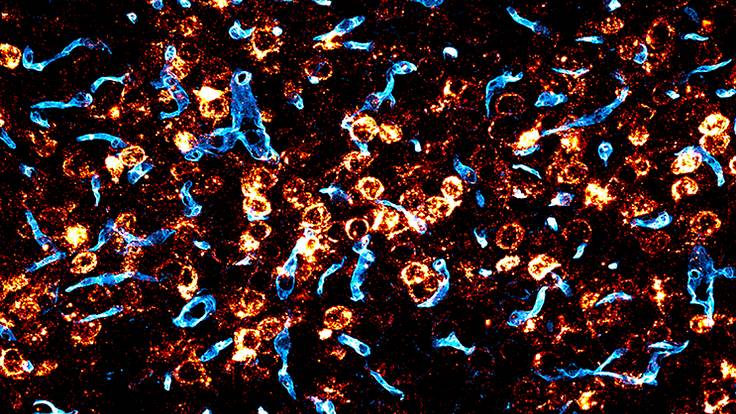
Researchers from the Broad Institute of MIT and Harvard have taken a significant stride toward more effective gene therapies for brain diseases by engineering a gene-delivery vehicle that efficiently crosses the blood-brain barrier in mice. The vehicle, an adeno-associated virus (AAV), uses a human protein to navigate this formidable obstacle, offering hope for future therapies in patients.
The blood-brain barrier, a highly selective membrane separating the blood from the brain, has long hindered the development of safer and more effective gene therapies for brain disorders. While gene therapy holds the potential to treat a range of severe genetic brain diseases that currently have no cures and few treatment options, FDA-approved forms of the most commonly used delivery vehicles struggle to efficiently cross this barrier and deliver therapeutic cargo.
Targeting a Human Protein
Ben Deverman and his team at the Broad Institute have engineered the first published AAV that targets a human protein to reach the brain in humanized mice. The AAV binds to the human transferrin receptor, which is highly expressed in the blood-brain barrier in humans. In their study, published in Science, the researchers demonstrated that their AAV, when injected into the bloodstream of mice expressing a humanized transferrin receptor, crossed into the brain at much higher levels than AAV9, the AAV used in an FDA-approved gene therapy for the central nervous system.
The engineered AAV also reached a large fraction of crucial brain cells, including neurons and astrocytes. The team further showed that their AAV could deliver copies of the GBA1 gene, linked to Gaucher’s disease, Lewy body dementia, and Parkinson’s disease, to a significant portion of cells throughout the brain.
Potential for Treating Brain Disorders
The scientists suggest that their new AAV could be a better option for treating a wide range of brain disorders, including neurodevelopmental disorders caused by single-gene mutations, such as Rett syndrome and SHANK3 deficiency; lysosomal storage diseases like GBA1 deficiency; and neurodegenerative diseases such as Huntington’s disease, prion disease, Friedreich’s ataxia, and single-gene forms of ALS and Parkinson’s disease.
“If this AAV does what we think it will in humans based on our mouse studies, it will be so much more effective than current options,” said Deverman, the study’s senior author.
The team’s approach to finding a delivery vehicle with a greater chance of reaching the brain in people involved screening a library of AAVs in a test tube for ones that bind to a specific human protein, followed by testing the most promising candidates in cells and mice modified to express the protein. This method, published by the researchers last year, provides valuable information about how the AAVs reach their targets, making it easier to translate a gene therapy using these AAVs from animals to humans.
As researchers continue to refine and improve the gene-delivery efficiency of these engineered AAVs, the potential for transformative gene therapies for brain disorders grows ever closer. With a biotech company co-founded by Deverman already developing new therapies using these AAVs to target the central nervous system, the future of gene therapy for brain diseases looks brighter than ever.
